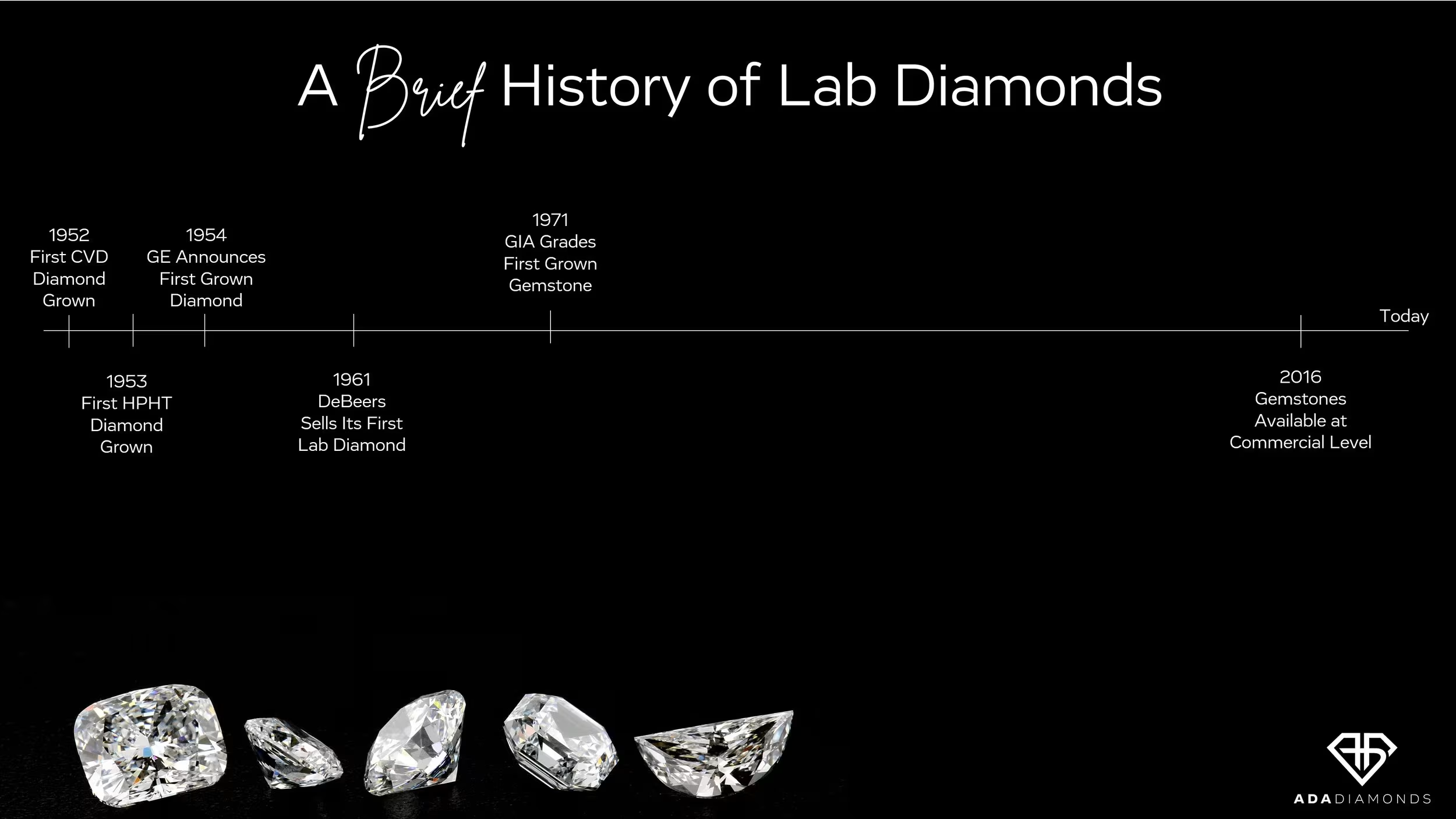
Lab diamonds are not a brand new technology. The first CVD and HPHT diamonds were grown in the 1950’s. De Beers has been selling lab diamond for industrial purposes for over 60 years. GIA first graded a lab diamond gemstone in 1971: a 0.30ct J VS1! It then took over 45 years of research and development for the technologies to mature enough for widespread consumer adoption.

Beyond the 4 C’s
GIA Guest Lecture Featuring Ada Diamonds Founders Lindsay Reinsmith and Jason Payne

- Differences between lab and natural diamonds
- Color tinges
- CVD diamond growth and BGS (brown, gray, strain & striations)
- HPHT diamond growth and BGP (blue, gray, phosphorescence)
- How and why crystal defects occur in lab diamonds
- How these defects are measured and determined
- Why you should care!

Overview of Lab Diamonds vs Natural Diamonds
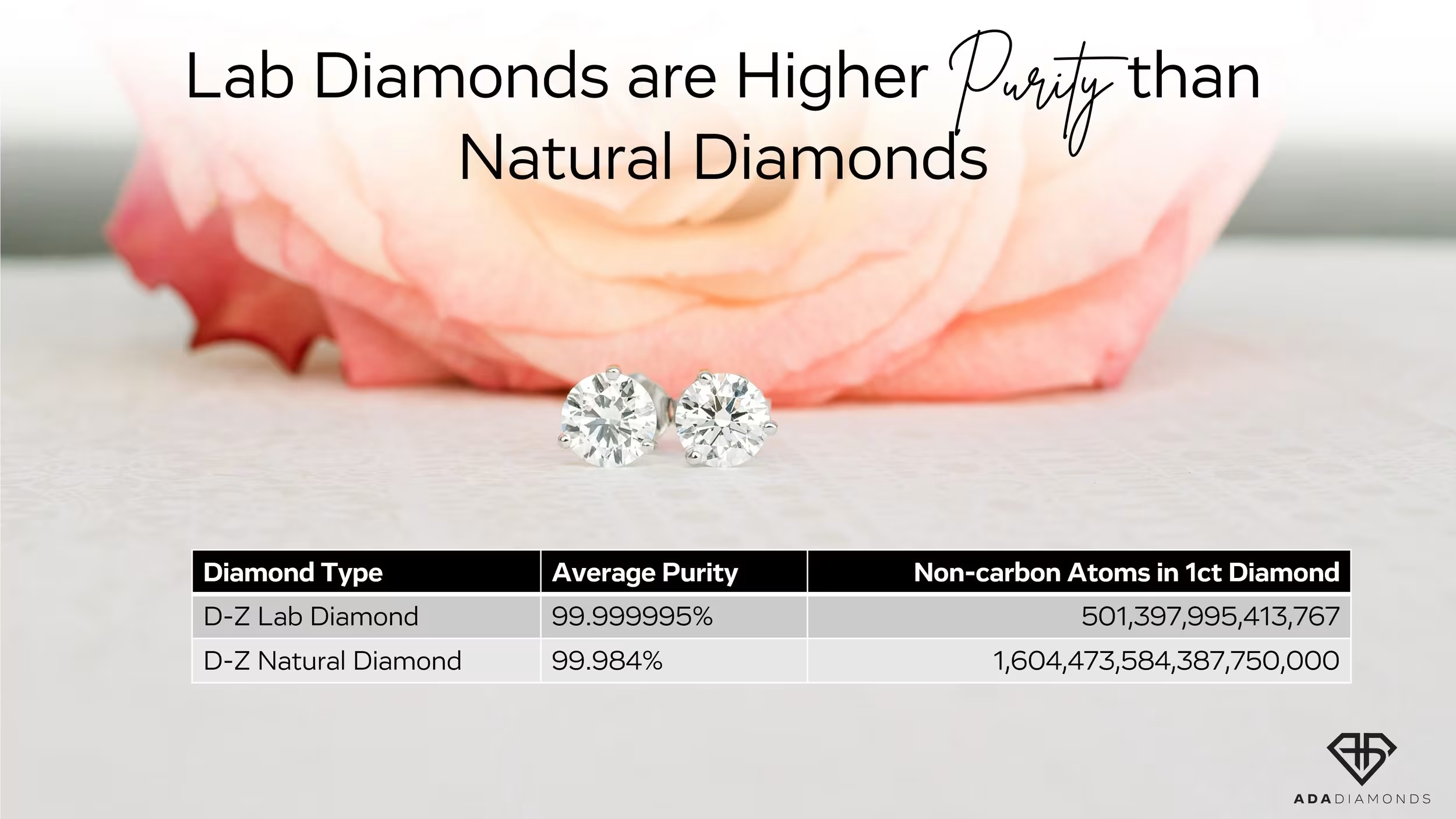
Lab Diamonds Have Fewer Impurities



Lab Diamonds Are Better and More Pure


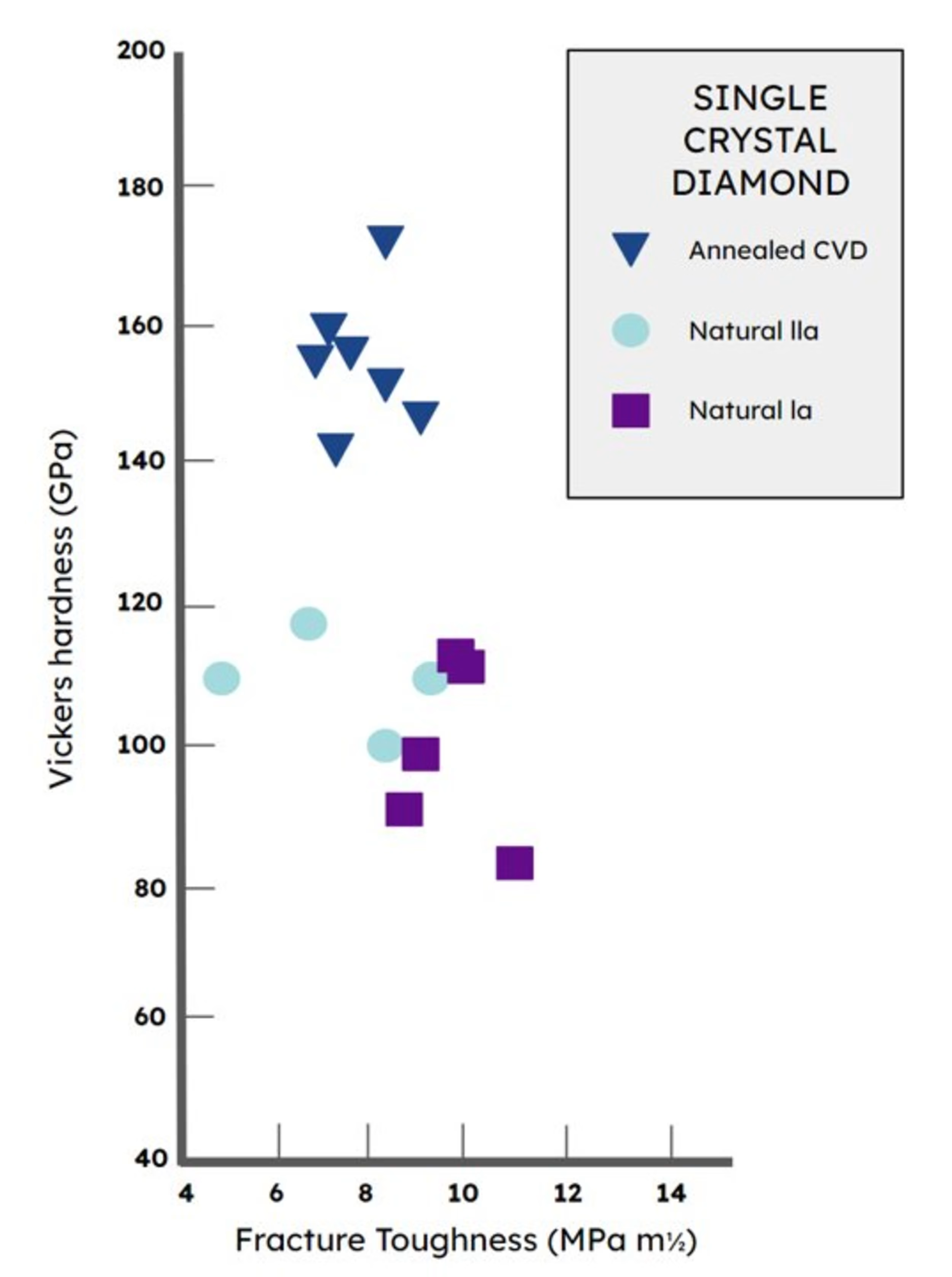
The quality of the best lab diamonds exceeds the quality of the best natural diamonds.
Ada’s lab diamonds are some of the most sensational on earth.
Some Defects in Lab Diamonds are Visible Without Any Special Tools
What Should You Look For in Evaluating Diamonds?

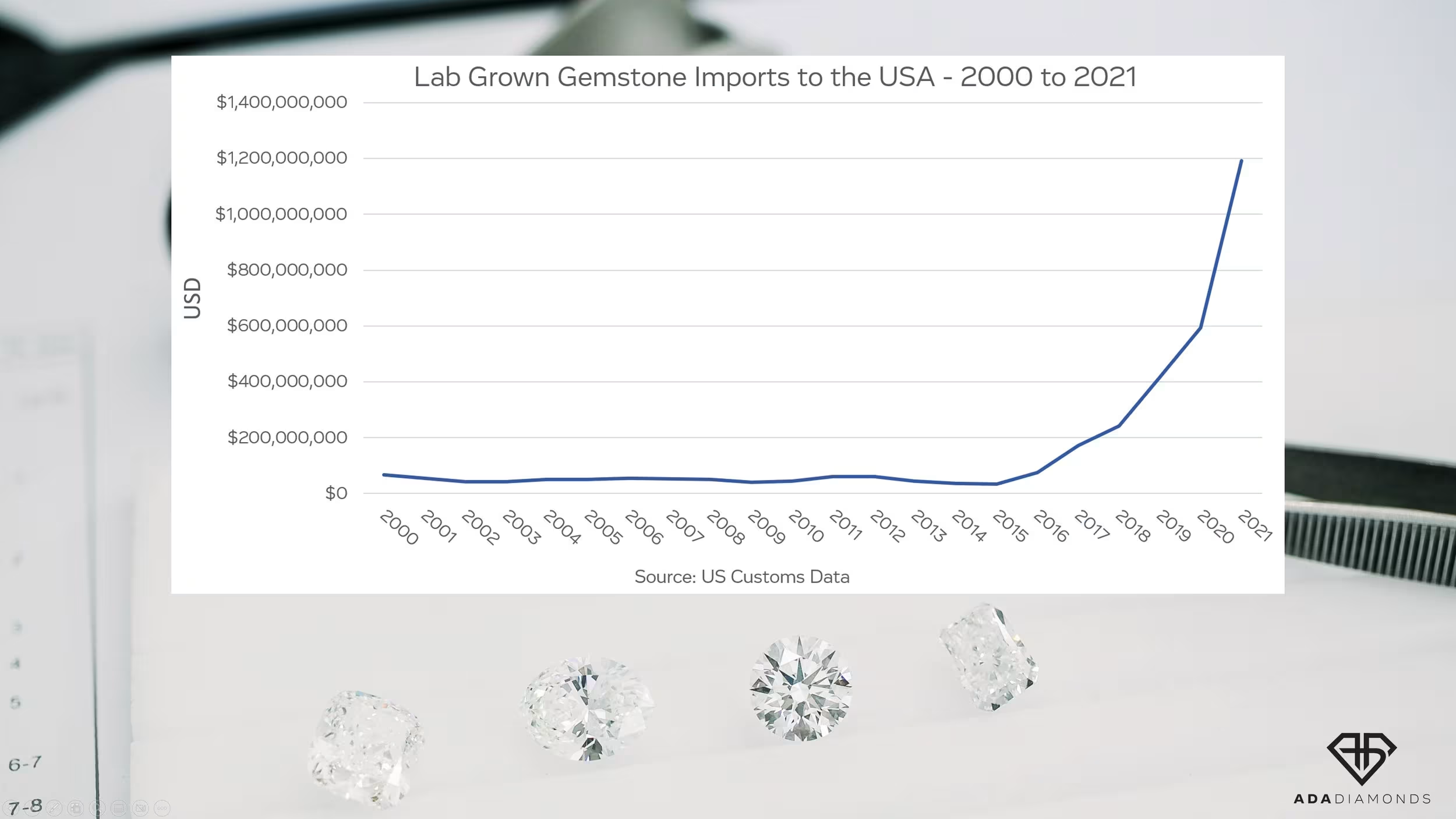
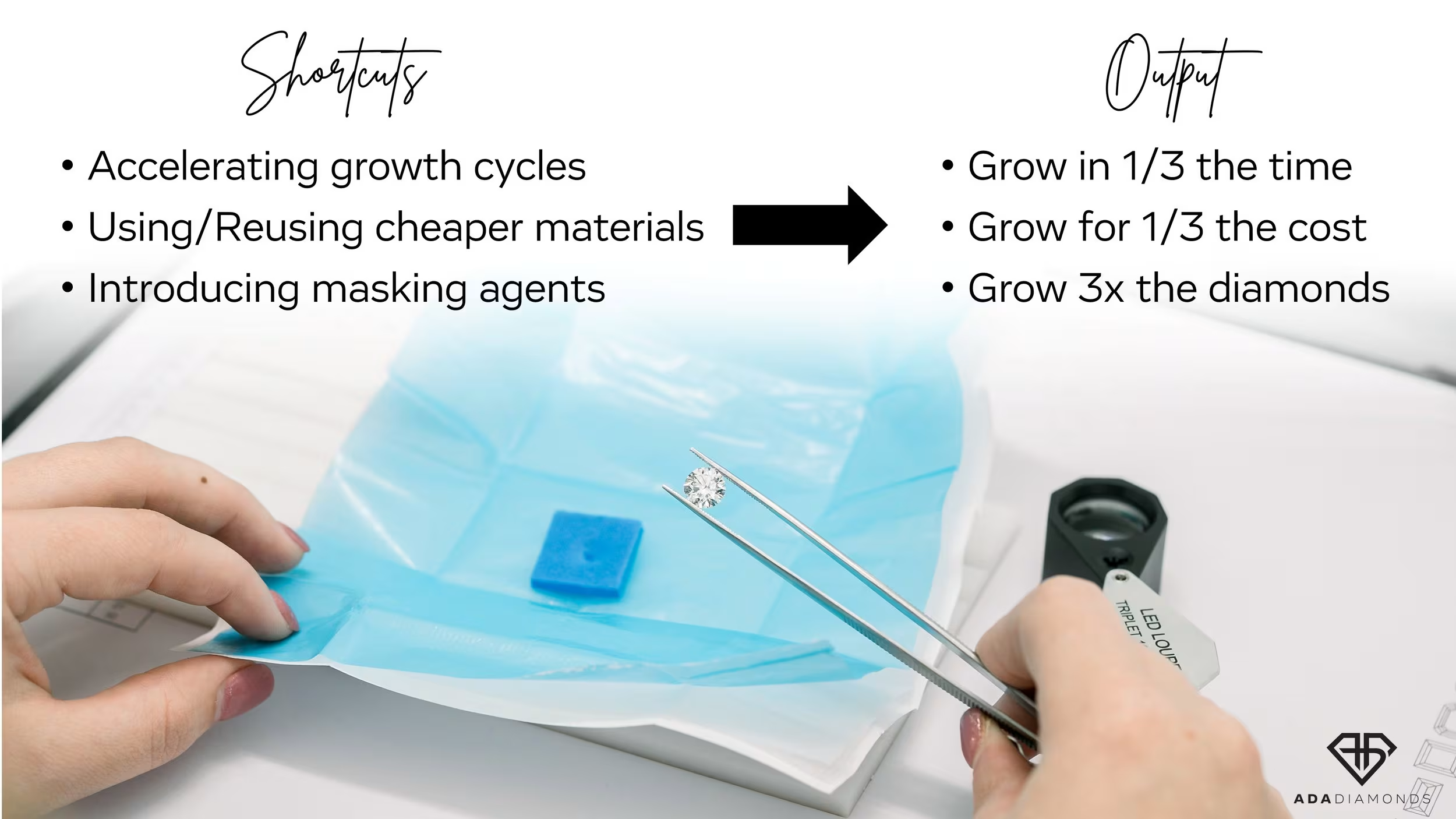
At the start of the commercialization of lab diamonds, there was a strong desire to produce a product indistinguishable from natural, but with higher purity. Over the last 3 years, the lab diamond industry has seen an explosion in demand as well as a significant increase in the number of CVD growers around the world. Nascent, aspirational growers purchased disadvantaged tech reactors to get in on the action. People who had no business being in this business entered the market, and many of them still don’t know what they’re doing.
The manufacturing side of diamonds couldn’t get enough. COVID-19 disrupted diamond mining far longer than it disrupted diamond growing. Existing CVD growers began getting pressured (pun intended) to produce more and more with their existing equipment without reinvestment. The goal: produce as much as possible for as cheaply as possible.


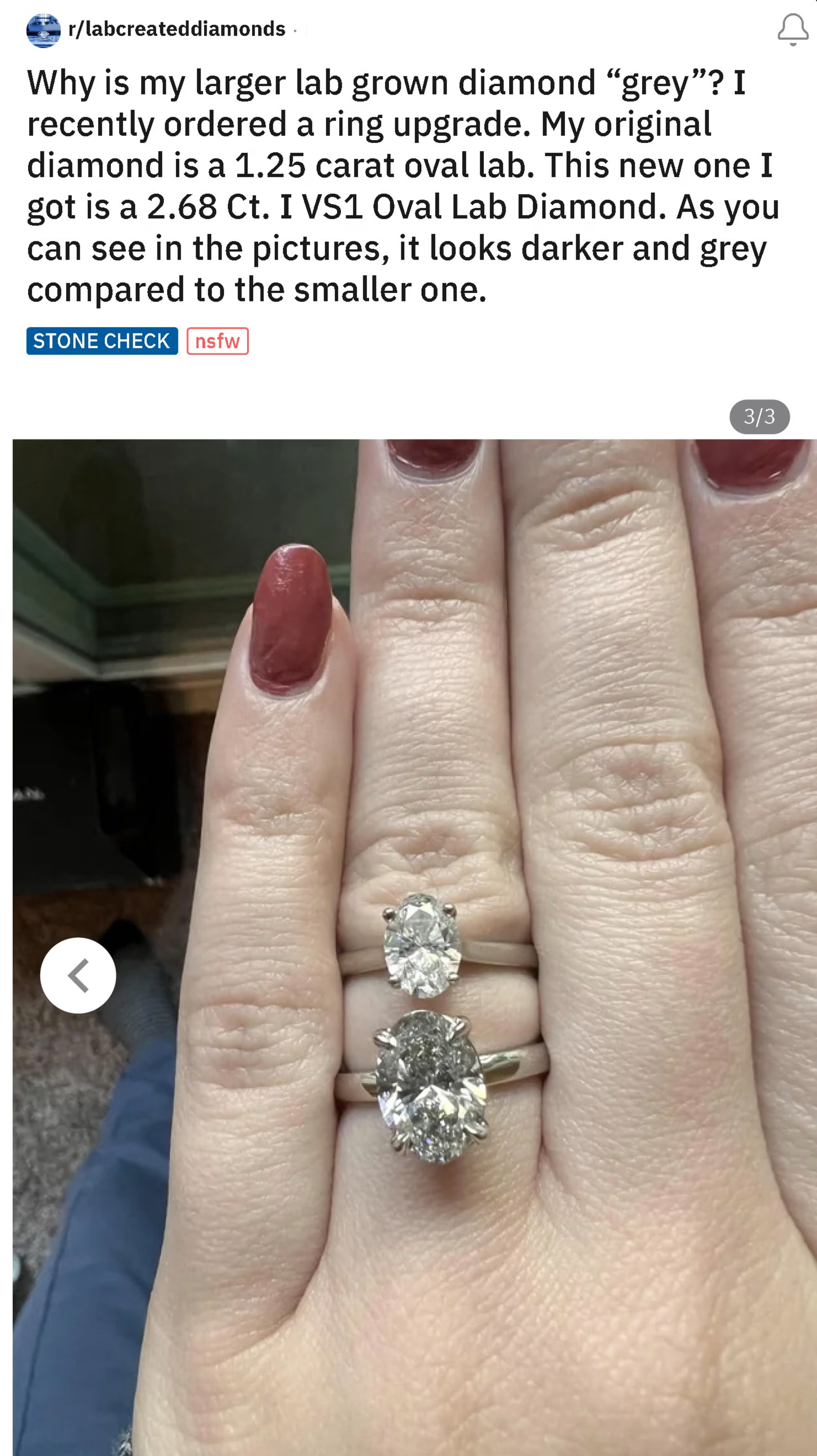
Here’s a lab diamond purchaser posting on Reddit asking why their new I color lab diamond is so gray compared to their other lab diamond.
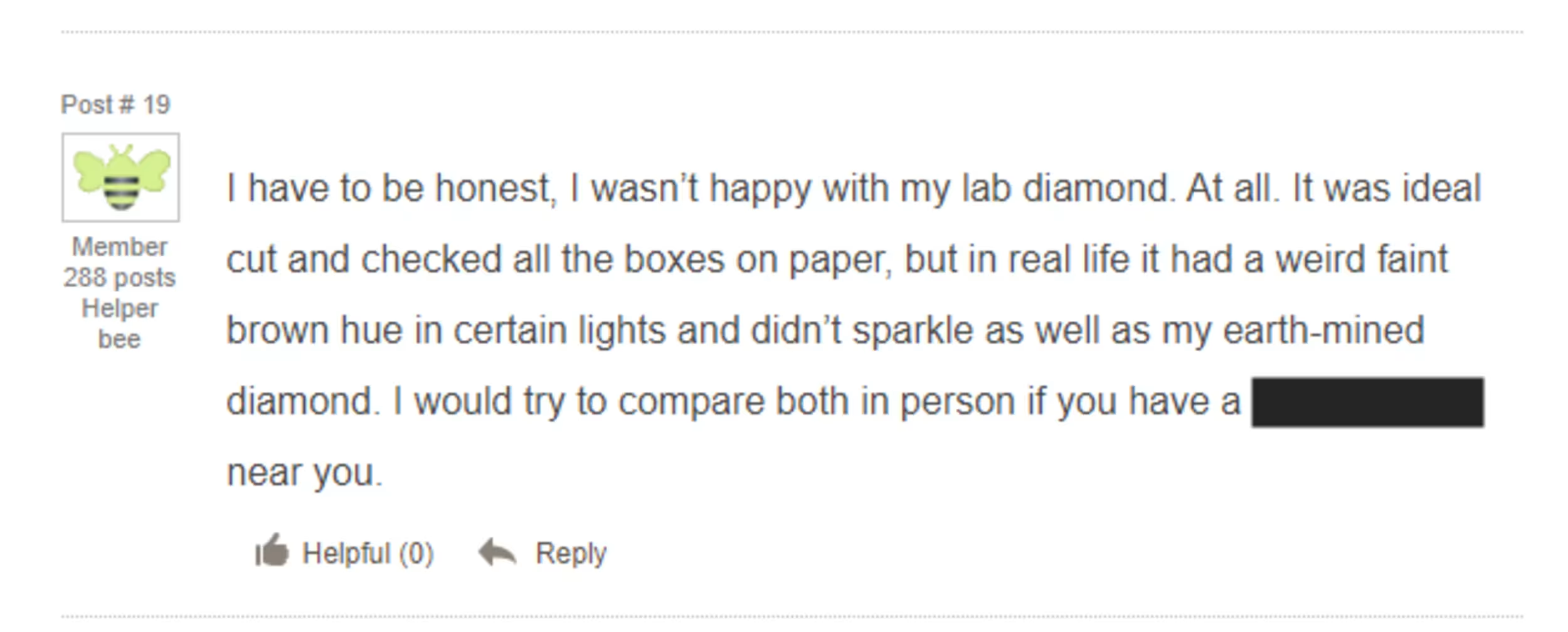
Here’s a lab diamond purchaser posting on Wedding Bee that their lab diamond had a “weird” faint brown hue compared to their natural diamond.
Sentiments like these harm consumer confidence in lab grown as a whole. But there are absolutely stunning and gorgeous lab diamonds out there that aren’t gray and aren’t brown. Consumers deserve that information and transparency to make better decisions.

Chemical Vapor Deposition(CVD)
How CVD is Grown
The first step of CVD diamond growth is preparation of the seeds. Great care needs to be taken to ensure the seed is as perfect as possible, as the quality of the seed determines the quality of the diamond. The seeds are strategically arranged on the growth platform in the reactor, and the all the air is sucked out of the reactor. A high power microwave generator is activated, and ultra pure methane is fed into the reactor. The microwave energy causes a plasma to form above the seeds, which turns the seeds a bright red color.
In the plasma, the methane begins to break apart, and the carbon from the methane attaches to the diamond seeds, growing vertically, atom by atom, for weeks at a time. On the edges of the diamonds, black polycrystalline material begins to form. That black material is removed with a laser, and the gemstone can be cut from the rough CVD.
Approximately 80% of CVD diamonds undergo post-growth treatment, which is a final stage of refinement. The CVD diamond is loaded into an HPHT press for a quick 15-30 minute heat treatment to improve the crystallographic structure and appearance of the diamonds.
Video courtesy of ALTR Created Diamonds
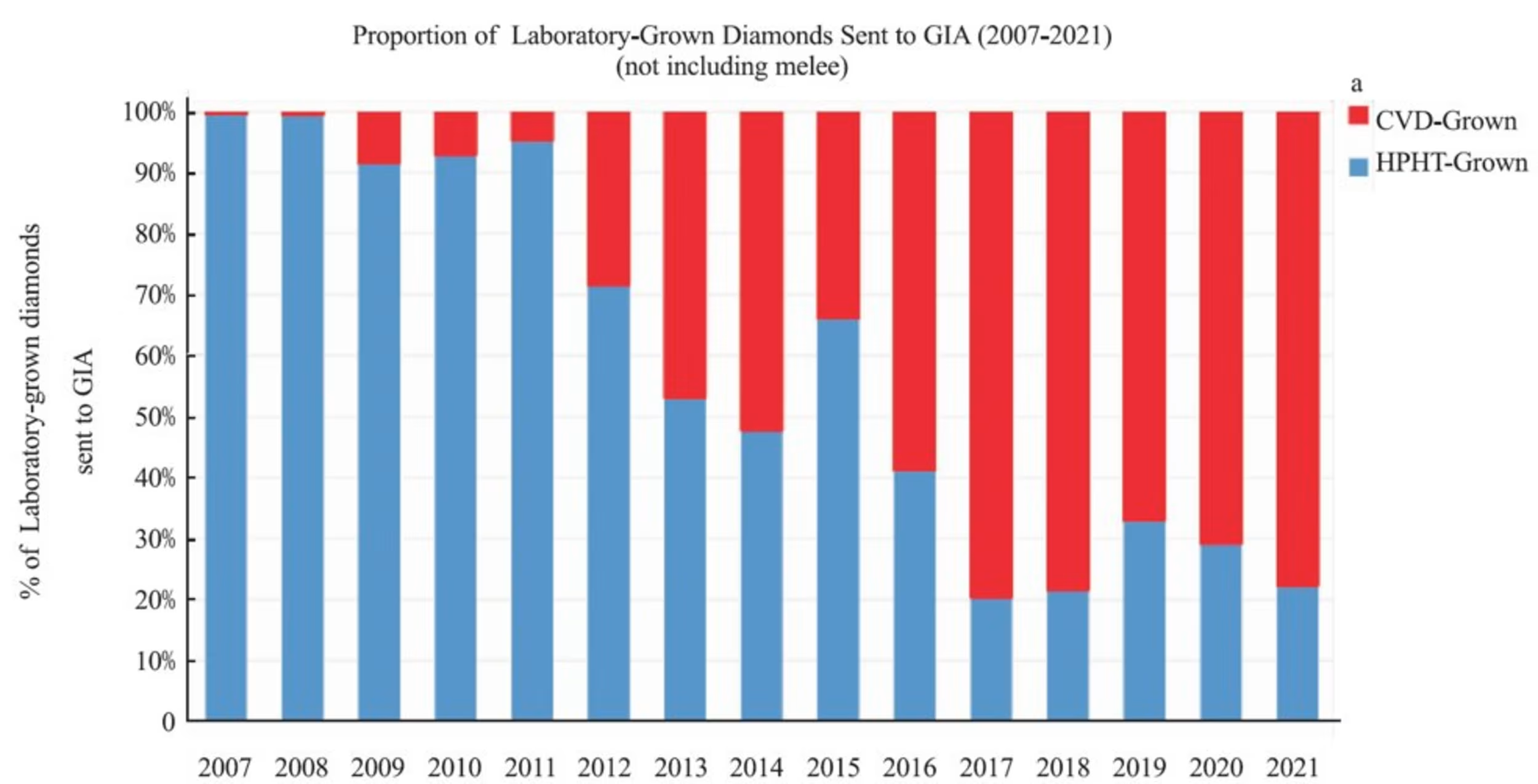
Since 2009, GIA has seen a steady increase in CVD grown diamonds to approximately 80% of the total lab diamond grading reports in the latest published data.

Why is CVD outpacing HPHT? It’s a more efficient technology. The equipment is substantially smaller and does not require a specialized facility. It generally produces Type IIa completely inert under SW and LW light and does not test as moissanite (for the most part) on cheap diamond handheld diamond testers. It’s also possible to observe diamond growth and restart growth when problems arise
What are the drawbacks and limitations of CVD? It has slower growth rates than HPHT, requires expensive seeds from third parties, and quality can vary. CVD is more prone to crystal strain, which can cause blurriness. It’s also less economical than HPHT for melee sized diamonds, also known as accent diamonds. Most HPHT melee is high color, which means pairing a much warmer CVD with bright white side stones can be visible to the naked eye.
G Color CVD Lab Diamonds
These are all G color CVD lab diamonds. As you can see the color tinges can vary in hue, saturation, and severity, even within the same color grade.
Brown hues in CVD diamonds can be more of a pinkish brown or more grayish brown. Both of those diamonds are from the same grower! Brown tinge also occurs in natural diamonds, though the reason for brown in natural diamonds is sometimes due to a completely different type of defect (plastic deformation).
In CVD diamonds, brown is caused by two main things:
Nitrogen defects can be accidental, leaking into the reactors past faulty seals, or nitrogen can be intentionally added as a catalyst to speed up the growth process.
Small amounts of nitrogen allow you to grow diamonds 200-300% faster. However, the faster you grow CVD diamonds, the more that can go wrong. As the atoms stack on top of each other, sometimes there are stacking faults, or glitches in the matrix of carbon atoms. One of the issues that can arise are voids of missing carbon in the crystal, like bubbles trapped in ice.
To demonstrate a void in CVD diamond, we’ve effectively sliced the carbon lattice in half and painted the cut line green. You can see that atoms are missing from the center of the lattice – that’s the void. When light hits a void in a diamond, the result is a brown coloration of the diamond.
Nitrogen Defects

Nitrogen can also give CVD diamonds a brown tone. Even though there is far less nitrogen in a CVD diamond than an average natural diamond, the optical consequences of said nitrogen can be more obvious to the naked eye in CVD than in natural. Single substitutional atomic nitrogen is actually more apparent and brown in tone than aggregated nitrogen.

Brown can also mean brownish pink, and this is primarily caused by nitrogen vacancy defects resulting from low pressure, high temperature treatment.
If the color of as-grown CVD diamond is brown, it can be made colorless through post growth treatment. That treatment can remove the voids from the crystal structure and also aggregate the nitrogen atoms, reducing the amount of brown they create.

A more extreme example of untreated (left) versus treated (right) CVD.
G = Gray
The most prevalent color tinge is gray and it can have several causes. It may also be accompanied by lifeless material. Gray is also possible in natural diamonds, but for the most part not at the same level as in lab grown and certainly not for the same reasons. Gray tinge can be seen as high as D color.
Both F Colors
The diamond on the right has a gray tinge over the diamond on the left.
Both D Colors
The diamond on the left has a gray tinge over the diamond on the left.
Causes for Gray: Silicon Vacancy Defects

Silicon trapped in the crystal can cause gray tinges. Silicon is very rare in natural diamonds, but common in CVD diamonds. Most frequently, the silicon is from the inside of the quartz window of the CVD reactor literally burning off into the vacuum chamber from the plasma.
Causes for Gray: Boron Compensating for Nitrogen
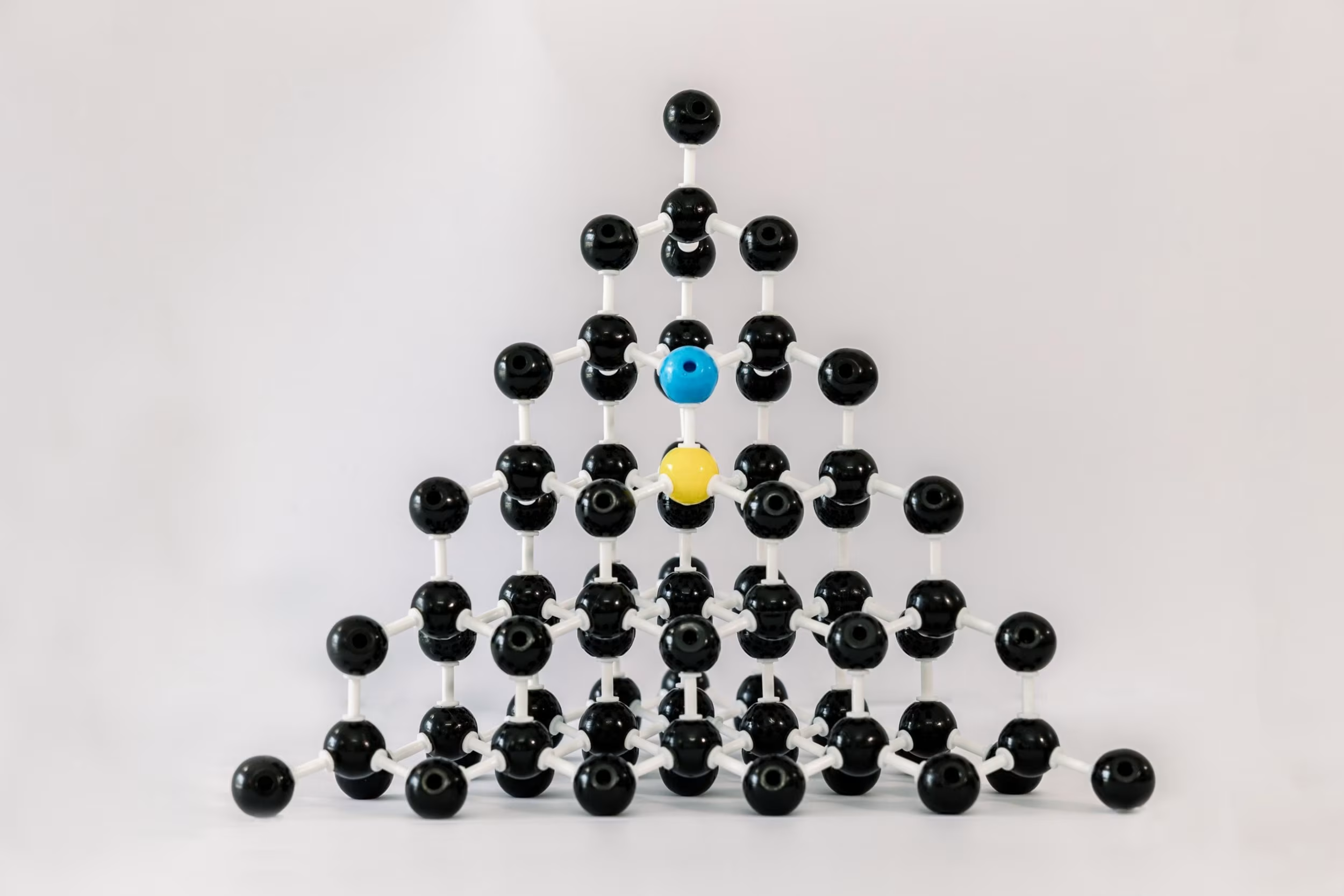
Adding nitrogen speeds up the diamond growth, but it leaves an undesirable brown tinge. You can compensate for the nitrogen by adding boron to your recipe – the pairs of atoms find each other and cancel out the properties of each other. But if you add too much boron, the nitrogen is overruled, and the diamond can present with a gray tinge/tone.
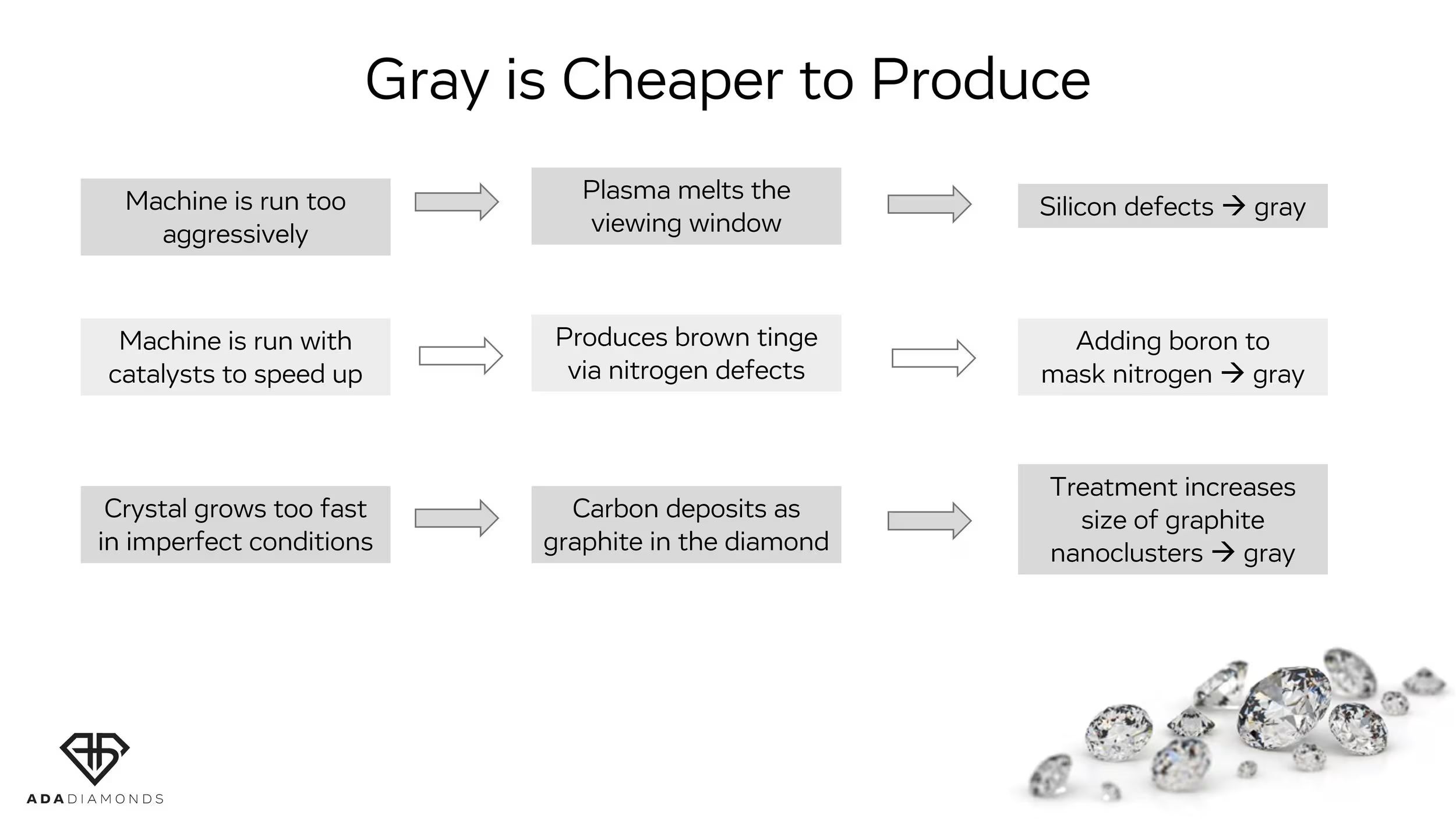
Causes for Gray: Graphitic Nanoclusters
Gray can also be the result of graphite nanoclusters, which is essentially carbon forming inside the crystal as little chunks of graphite instead of as diamond. The faster you run your reactor, the more likely it is you get graphite clusters trapped in the diamond. Graphite cannot be treated out. Moreover, graphite provokes formation of more graphite at high temperatures. Thus, if gray color component is present, this gray color can get worse after high temperature treatment.
Gray Can Get Worse with Treatment
When the void collapses, the graphitic inclusion nearby can expand into the space that used to be occupied by the void, causing a stronger gray tinge. Bottom line: removing the brown and/or strain from a CVD diamond can make it more gray!
S = Strain

CVD Strain Often Looks Like Streaks on Glass
Strain presents as blurriness in the crystal, and it looks almost like you can never get it fully clean. The closest approximate natural characteristic is graining.
CVD Strain Causes Light to Scatter
You can see the “shimmer” on the CVD rough as the light hits the strain.
Strain is Not a Binary

Strain is not measured as a binary but rather exists on a scale of good to bad. There’s little to no consensus in the industry as to what is light or faint and what is strong. Strain is not a clarity setting feature, meaning it does not appear in inclusion maps or change clarity grades. All of the above diamonds are VVS in clarity.
Crystal Strain Looks Different Depending on the Type of Diamond
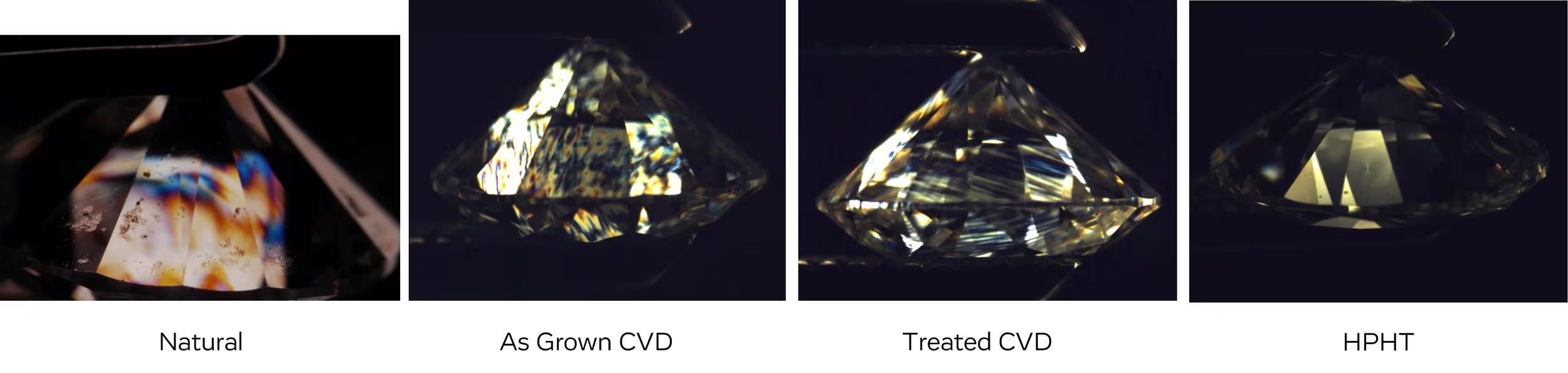
Another reminder that lab diamonds are not identical to natural and can be detected! It’s worth noting that HPHT has little to zero strain when viewed under cross polar filters.
Strain Starts with Seeds
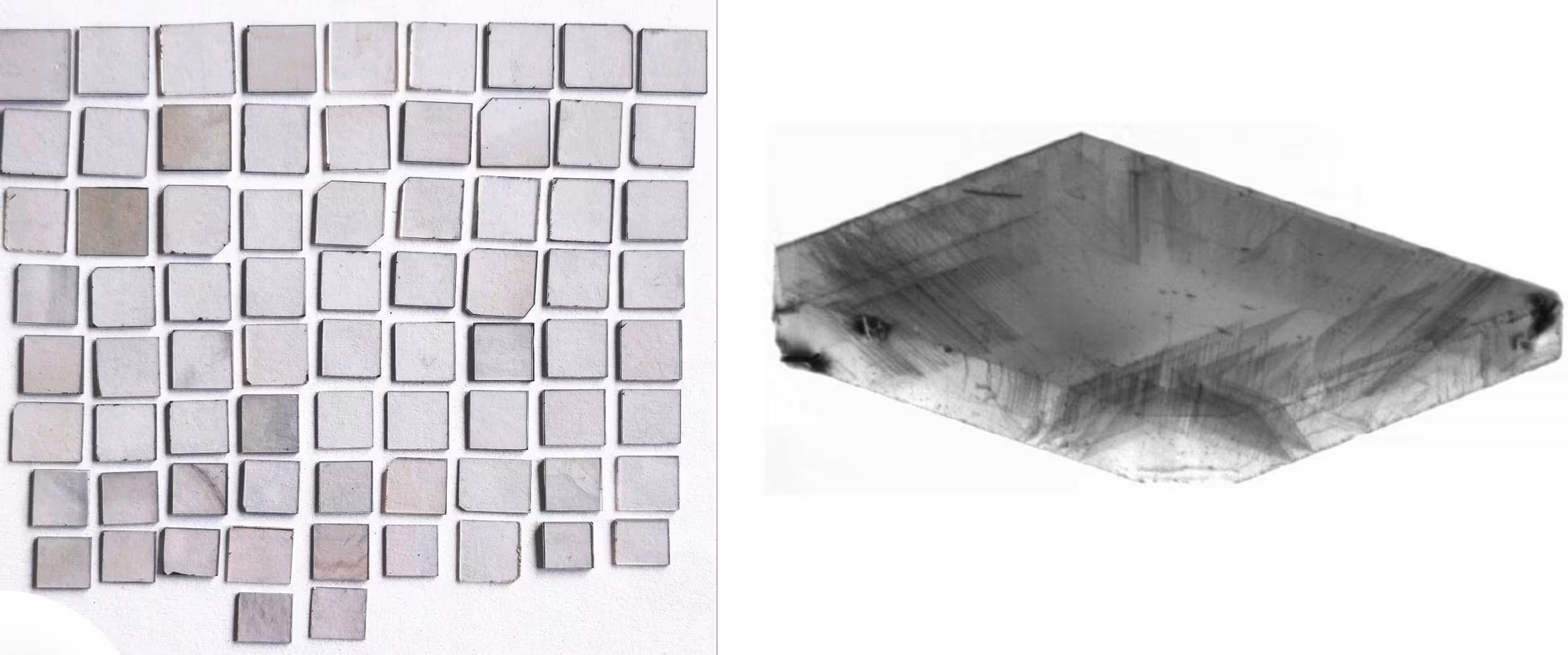
The quality of seeds can vary significantly and build either a strong or weak foundation for CVD diamond growth on top. Seeds viewed through an x-ray (at right) can show faults or uneven surfaces.
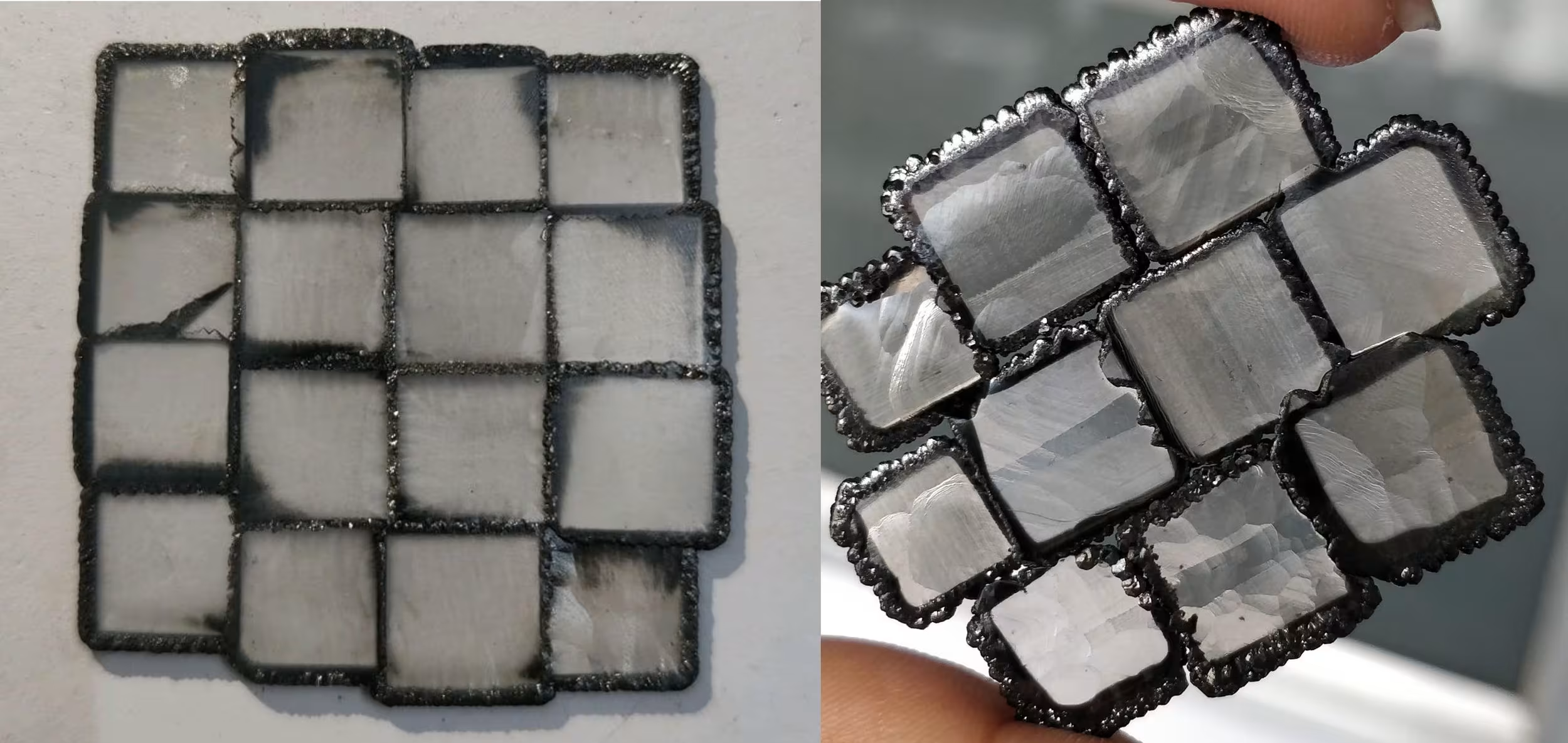
One CVD reactor can have varying degrees of quality seeds. The quality of the material of the grown CVD can vary significantly for the same grower and even in the same growth run!

Seeds deteriorate with each use and the diamonds grown on top of them get worse and worse. Replacing seeds is very expensive. Many growers will use and reuse seeds far past their prime.
Post-Growth Treatment Heals Strain

Strain Accumulates Until Polycrystalline Nucleates
As strain builds, it can eventually explode, almost like a volcano, and form into polycrystalline inclusions. That does impact clarity. The diamond on left is CVD and diamond on right is HPHT. Both got the same clarity grade of I1.
You stop your machine, take the crystal out, laser off the polycrystalline layer, and put the diamond back into the reactor. What forms: striations.
S = Striations

Image at left courtesy of GIA
The impact of starting and stopping the CVD reactor are rings of diamond growth known as striations. Nearly all CVD diamonds have some striations, it’s not a binary.
The image at left shows 8 growth cycles, designated by obvious rings. It’s not just the presence of these growth rings. How far apart or close together the rings are represent a timetable showing how long the growth cycles lasted in between stops. Striations can make a diamond look out of focus and lackluster.
The diamond on the bottom has over 8 growth cycles and is more heavily striated. The diamond on the top has a cleaner crystal material. Note the difference in contrast between the two diamonds.
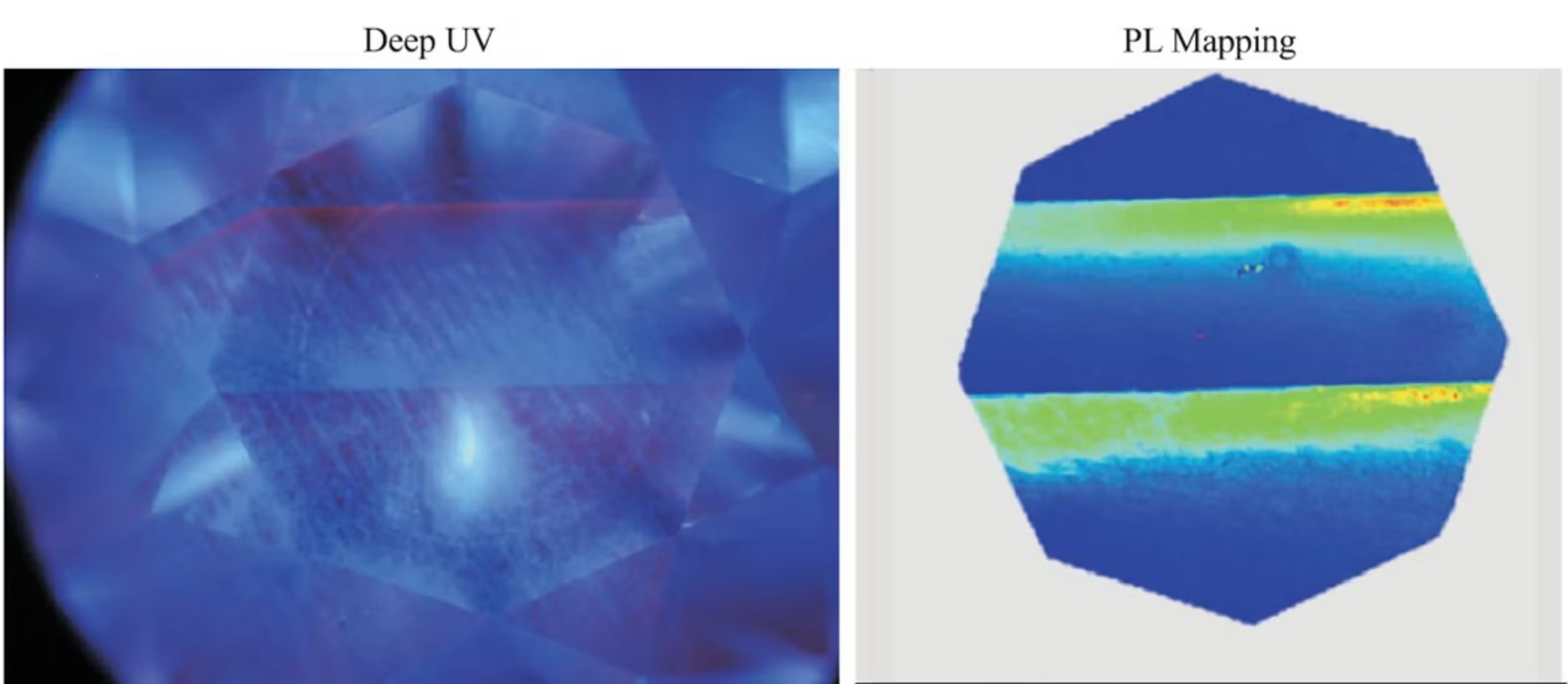
Every time a CVD reactor is stopped and restarted, contaminants get into the plasma, which cause the rings of diamond growth to look dark gray, further contributing to color tinge face up.
This was a G color CVD diamond we sent for additional analysis to GIA’s researchers. It has a very obvious gray tinge. After spectral analysis, we concluded this was likely due to a combination of silicon defects and graphitic nano inclusions. The silicon and silicon vacancy defects were too significant to have been from the CVD viewing glass, and were likely the result of intentionally added silicon during growth. The zoning and clear growth line (shown in bright green) indicate that this diamond was likely moved from one reactor to another generation CVD reactor with a different chemistry profile. It’s possible one of the reactors was being used for R&D purposes.

Approximately 80% of GIA graded CVD diamonds are HPHT treated. Why? Proper HPHT treatment makes the diamonds better. Period.
We believe the resistance to post growth treatment is a hangover effect from the natural diamond market where there’s legitimate concern about undisclosed manmade treatment being done to a natural product. But in lab grown, the product is manmade throughout the entire process. And what one grower considers treatment another may consider simply part of the growth process.
Untreated CVD has some flaws. It’s less durable and has more crystal material issues like brown and strain. Post-growth treatment effectively heals brown and strain. Not all treated CVD is good, and not all of it is bad.

High Pressure High Temperature (HPHT)
How HPHT is Grown
Small diamond seeds are placed at the bottom of a growth cell. Catalytic metal material is placed on top. Graphite is added into a gasket. This growth cell is then placed at the center of a hydraulic press where six different anvils exert pressure on six different sides (which is why it’s called a cubic press). The press is heated to 1500 degrees Celsius and subjected to 1 million psi of pressure. The graphite is heated to the point where it melts into liquid carbon. A convection is created. As the press cools, the carbon affixes to the diamond seeds and grows atom by atom, crystalizing into diamond. The seeds with which the diamonds are grown in the rough HPHT are visible upon removal.

HPHT is Prone to Unique Color Tinges
In evaluating HPHT diamonds, we recommend looking for BGP:
B = Blue
Blue tinge in HPHT diamonds is a very common crystal defect. This is due to trace boron that is intentionally added during the growth process to compensate for nitrogen. Measurable levels of boron are considered Type IIb. 75% of HPHT diamonds submitted to GIA for grading are Type IIb, but that doesn’t necessarily mean that they all have blue tinge! Type IIb is more likely to have blue tinge, but it’s not definitive.
In the video above, the D, F, and H color HPHT diamonds have a blue tinge, but notice that the E color does not. Color is measured for the presence of any color, not just yellow.
While there certainly exist blue natural diamonds, they are exceptionally rare, making up less than 0.1% of the gemstone market and fetching significantly higher prices per carat than traditional natural or lab diamonds. It is not feasible that a member of the public would “accidentally” purchase a large Type IIb natural diamond. So having a blue tinge diamond may make someone question whether the material was diamond or something else like aquamarine or moissanite.
While a blue tinge may be a consumer’s personal preference, it is technically a crystal defect.

Both “F” color HPHT lab diamonds. The bottom diamond has a blue tinge.
Why Blue Happens

For years, HPHT diamonds were yellowy orange due to the presence of nitrogen. Added boron helps to compensate for the nitrogen. In some cases you can end up with excess nitrogen, just the right compensation, or too much boron. While this practice also exists in CVD, it’s much more common and at a more significant level in HPHT.
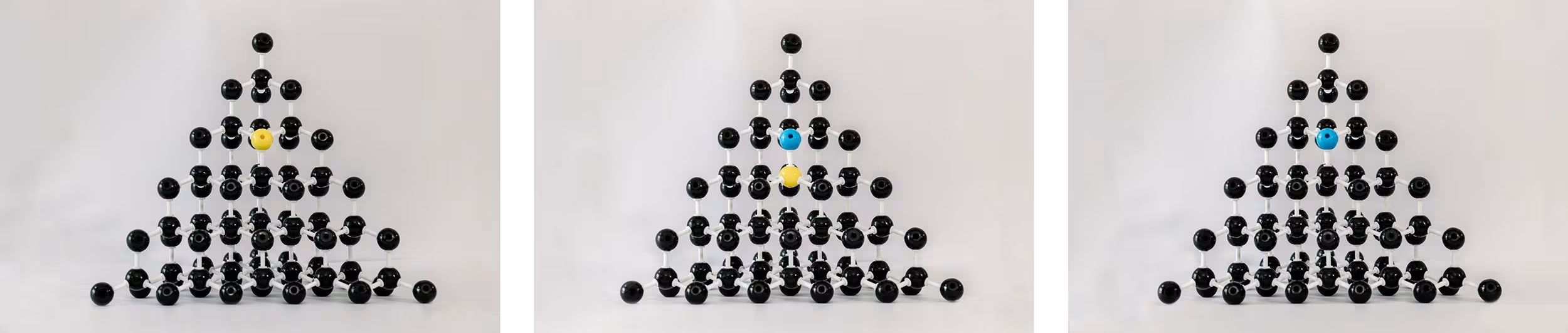
Left to right: Substitutional nitrogen atom; boron compensating for nitrogen; excess boron.
During HPHT growth, you can have some diamonds that are blue and some that aren’t, and even within the diamond you can have some parts of the diamond that are blue and some that are not.
This is the spectral analysis of a single grower of HPHT. You can see in some cases the boron spikes are very low and in others quite high. Even though nitrogen is more likely to be dispersed throughout a diamond’s crystal lattice, boron can be growth sector dependent and quite concentrated. In other words, boron is very difficult to control.
This type of boron zoning would not be present in a natural Type IIb diamond.
In some cases you can have both a growth sector of nitrogen and a growth sector of boron. In this case, this greenish tinge pear had both, resulting in blue + yellow = green. Despite having enough nitrogen to modify the color, this HPHT diamond is still Type IIb based on the boron content as there is more boron than nitrogen. This is NOT a high enough concentration to be a fancy color diamond. It received an “I” color grade.
G = Gray

Gray HPHT can have nice material but a slate color
There are lots of reasons why HPHT can look gray. For some, it’s simply that it has a blue tinge that looks gray in certain lights. Others can be irradiated or have elements like aluminum, titanium, nickel, or other trace metals. Some can have metallic inclusions.
The exact formulas that keep out nitrogen are tightly held trade secrets. Those secrets can determine if HPHT diamonds are grayish blue or bright white.
This is a fascinating diamond we shared with GIA. In our conversations with GIA researchers, we’re not 100% certain why it has this super obvious gray tinge. One theory is that it’s due to the presence of growth tubes, seen here under SW light as uniform streaks or claw marks. This diamond is also Type IIb, had strong phosphorescence, but does not appear blue in person. This is honestly one of the ugliest lab diamonds we’ve ever seen. Super interesting.

P = Phosphorescence
Phosphorescence can be seen in some HPHT diamonds after exposure to long wave light. Phosphorescence can have zoning just like boron.
Phosphorescence is:
We’re only discussing phosphorescence under long wave UV light and sunlight. Shortwave light is filtered by our atmosphere and using it at high power can be dangerous and should only be done by trained professionals.
Here are four HPHT diamonds. On the far left, there’s one that has a blue tinge and one that has a yellowy tinge. The yellowy tinge emerald cut is Type IIb despite not having a blue tinge. On the right there’s a blue tinge as well as a yellowy tinge HPHT stone.
The four diamonds are moved from the paper tray to a perforated tray. After just a few seconds of long wave UV light using a handheld light from Amazon (you can buy for $5) being applied, the main light is turned off. The two diamonds on the left had very strong and moderate phosphorescence and the two diamonds on the right are inert, with no reaction to long wave UV light.
Phosphorescence is not just seen in blue tinge or avoidable in yellowy tinge. It is connected to boron at an atomic level, not an optical one.
Well, Thats Interesting
These same four diamonds were left in the office for about an hour. Once the light was turned off, the diamonds glowed again. We concluded this was not, in fact, prolonged phosphorescence, but merely phosphorescence that had been activated by the office lighting. So it doesn’t take special equipment, gemological tools, or even sunlight to activate phosphorescence. You just need darkness to see it.

The office light that activated phosphorescence.

Why This Matters
Phosphorescence is not disclosed on a grading report and is only something that can be tested for in person. It’s also not a great “surprise.” Imagine walking into a dark movie theater and suddenly seeing your diamond glow, without warning. Consumers believe that lab diamonds are chemically identical to natural diamonds, but the number of natural diamonds that have phosphorescence under normal indoor lighting? Fewer than 0.1%. The presence of glow-in-the-dark diamonds has the potential to erode consumer trust in HPHT lab diamonds if consumers don’t know that it exists. Medium to strong phosphorescence can also make a lab diamond look cloudy/hazy in low light environments.

Ada Diamonds is the Foremost Authority on Lab Diamond Quality

I have personally inspected over 15,000 certified lab diamonds above 1 carat. I’ve seen diamonds from over 100 growers and 10 different countries. I look at lab diamonds side by side every week. And I’ve been documenting and cataloging my assessments over the last seven years.
The breadth of quality I’ve seen makes me uniquely qualified to speak about this product and what consumers should look out for. Despite my exposure and expertise, I would never, ever, ever buy a lab diamond totally sight unseen. And I don’t recommend that you do, either.
I want to remind the public that lab diamonds are, fundamentally, superior to natural diamonds in their crystal purity. There is no denying that. Beyond purity and the 4 C’s, there are lab diamonds that rival the absolute best natural diamonds in the world. Impeccable crystal material, bright white shine, and near perfect cut.
But there are also lab diamonds out there that are defective enough to make you question if it’s a diamond at all.
How consumers can protect themselves:
1. Work with a jeweler that you trust.
2. Don’t rely simply on a grading report or a low resolution video.
3. Compare lab diamonds side by side.
4. And when it comes to price, if it seems too good to be true, it probably is.
-Lindsay Reinsmith, Founder, Ada Diamonds